Bohr,
Niels Henrik David
Bohr, Niels Henrik David (1885-1962), Danish physicist and Nobel
laureate, who made basic contributions to nuclear physics and the understanding
of atomic structure.
Bohr was born in Copenhagen, the son of a physiology professor,
and was educated at the University of Copenhagen, where he earned his doctorate
in 1911. That same year he went to Cambridge, England, to study nuclear physics
under British physicist Sir Joseph John Thomson, but he soon moved to
Manchester to work with another British physicist, Ernest Rutherford.
Bohr’s theory of atomic structure (see Quantum Theory), for
which he received the Nobel Prize in physics in 1922, was published in papers between
1913 and 1915. His work drew on Rutherford’s nuclear model of the atom, in
which the atom is seen as a compact nucleus surrounded by a swarm of much
lighter electrons (see Atom). Bohr’s atomic model made use of quantum
theory and the Planck constant (the ratio between quantum size and radiation
frequency). The model posits that an atom emits electromagnetic radiation only
when an electron in the atom jumps from one quantum level to another. This
model contributed enormously to future developments of theoretical atomic
physics.
In 1916 Bohr returned to the University of Copenhagen as a
professor of physics, and in 1920 he was made director of the university’s
newly formed Institute for Theoretical Physics. There Bohr developed a theory
relating quantum numbers to large systems that follow classical laws, and made
other major contributions to theoretical physics. His work helped lead to the
concept that electrons exist in shells and that the electrons in the outermost
shell determine an atom’s chemical properties. He also served as a visiting
professor at many universities.
In 1939, recognizing the significance of the fission experiments (see
Nuclear Energy: Nuclear Energy from Fission) of German scientists
Otto Hahn and Fritz Strassmann, Bohr convinced physicists at a scientific
conference in the United States of the importance of those experiments. He
later demonstrated that uranium-235 is the particular isotope of uranium that
undergoes nuclear fission. Bohr then returned to Denmark, where he was forced
to remain after the German occupation of the country in 1940. Eventually,
however, he was persuaded to escape to Sweden, under peril of his life and that
of his family. From Sweden the Bohrs traveled to England and eventually to the
United States, where Bohr joined in the effort to develop the first atomic
bomb, working at Los Alamos, New Mexico, until the first bomb’s detonation in
1945. He opposed complete secrecy of the project, however, and feared the
consequences of this ominous new development. He desired international control.
In 1945 Bohr returned to the University of Copenhagen, where he
immediately began working to develop peaceful uses for atomic energy. He
organized the first Atoms for Peace Conference in Geneva, held in 1955, and two
years later he received the first Atoms for Peace Award. In 1997 the
International Union of Pure and Applied Chemistry announced that the chemical
element with the atomic number 107 would be given the official name bohrium
(Bh), in honor of Niels Bohr.
Microsoft
® Encarta ® Reference Library 2003. © 1993-2002 Microsoft
Corporation. All rights reserved.
Bohr,
Niels
b. Oct. 7, 1885, Copenhagen, Den.
d. Nov. 18, 1962,
Copenhagen
in full NIELS HENRIK DAVID BOHR
Bohr.
Danish physicist who was the first to apply the quantum theory,
which restricts the energy of a system to certain discrete values, to the
problem of atomic and molecular structure. For this work he received the Nobel
Prize for Physics in 1922. He developed the so-called Bohr theory of the
atom and liquid model of the atomic nucleus.
Bohr's father, Christian Bohr, professor of physiology at the
University of Copenhagen, was known for his work on the physical and chemical
aspects of respiration. His mother, Ellen Adler Bohr, came from a
wealthy Jewish family prominent in Danish banking and parliamentary circles. Bohr's
scientific interests and abilities were evident early, and they were encouraged
and fostered in a warm, intellectual family atmosphere. Niels's younger
brother, Harald, became a brilliant mathematician.
Bohr distinguished himself at the University of Copenhagen, winning
a gold medal from the Royal Danish Academy of Sciences and Letters for his
theoretical analysis of and precise experiments on the vibrations of water jets
as a way of determining surface tension. In 1911 he received his doctorate for
a thesis on the electron theory of metals that stressed the inadequacies of
classical physics for treating the behaviour of matter at the atomic level. He
then went to England, intending to continue this work with Sir J.J. Thomson at
Cambridge. Thomson never showed much interest in Bohr's ideas on
electrons in metals, however, although he had worked on this subject in earlier
years. Bohr moved to Manchester in March 1912 and joined Ernest
Rutherford's group studying the structure of the atom.
At Manchester Bohr
worked on the theoretical implications of the nuclear model of the atom
recently proposed by Rutherford and known as the Rutherford atomic model. Bohr
was among the first to see the importance of the atomic number, which indicates the
position of an element in the periodic table and is equal to the number of
natural units of electric charge on the nuclei of its atoms. He recognized that
the various physical and chemical properties of the elements depend on the
electrons moving around the nuclei of their atoms and that only the atomic
weight and possible radioactive behaviour are determined by the small but
massive nucleus itself. Rutherford's nuclear atom was both mechanically and
electromagnetically unstable, but Bohr imposed stability on it by
introducing the new and not yet clarified ideas of the quantum theory being
developed by Max Planck, Albert Einstein, and other physicists. Departing
radically from classical physics, Bohr postulated that any atom could
exist only in a discrete set of stable or stationary states, each characterized
by a definite value of its energy. This description of atomic structure is
known as the Bohr atomic model.
The most
impressive result of Bohr's essay at a quantum theory of the atom was the
way it accounted for the series of lines observed in the spectrum of light emitted by
atomic hydrogen. He was able to determine the frequencies of these spectral
lines to considerable accuracy from his theory, expressing them in terms of the
charge and mass of the electron and Planck's constant (the quantum of action,
designated by the symbol h). To do this, Bohr also postulated
that an atom would not emit radiation while it was in one of its stable states
but rather only when it made a transition between states. The frequency of the
radiation so emitted would be equal to the difference in energy between those
states divided by Planck's constant. This meant that the atom could neither
absorb nor emit radiation continuously but only in finite steps or quantum
jumps. It also meant that the various frequencies of the radiation emitted by
an atom were not equal to the frequencies with which the electrons moved within
the atom, a bold idea that some of Bohr's contemporaries found
particularly difficult to accept. The consequences of Bohr's theory,
however, were confirmed by new spectroscopic measurements and other
experiments.
Bohr returned to Copenhagen from Manchester during the summer of 1912,
married Margrethe Nørlund, and continued to develop his new approach to the
physics of the atom. The work was completed in 1913 in Copenhagen but was first
published in England. In 1916, after serving as a lecturer in Copenhagen and
then in Manchester, Bohr was appointed to a professorship in his native
city. The university created for Bohr a new Institute of Theoretical
Physics, which opened its doors in 1921; he served as director for the rest of
his life.
Through the early
1920s, Bohr concentrated his efforts on two interrelated sets of
problems. He tried to develop a consistent quantum theory that would replace
classical mechanics and electrodynamics at the atomic level and be adequate for
treating all aspects of the atomic world. He also tried to explain the
structure and properties of the atoms of all the chemical elements,
particularly the regularities expressed in the periodic table and the complex
patterns observed in the spectra emitted by atoms. In this period of uncertain
foundations, tentative theories, and doubtful models, Bohr's work was
often guided by his correspondence principle.
According to this principle, every transition process between stationary states
as given by the quantum postulate can be "coordinated" with a
corresponding harmonic component (of a single frequency) in the motion of the
electrons as described by classical mechanics. As Bohr put it in 1923,
"notwithstanding the fundamental departure from the ideas of the classical
theories of mechanics and electrodynamics involved in these postulates, it has been
possible to trace a connection between the radiation emitted by the atom and
the motion of the particles which exhibits a far-reaching analogy to that
claimed by the classical ideas of the origin of radiation." Indeed, in a
suitable limit the frequencies calculated by the two very different methods
would agree exactly.
Bohr's institute in Copenhagen soon became an international centre
for work on atomic physics and the quantum theory. Even during the early years
of its existence, Bohr had a series of coworkers from many lands,
including H.A. Kramers from The Netherlands, Georg Charles von Hevesy from
Hungary, Oskar Klein from Sweden, Werner Heisenberg from Germany, and John
Slater from the United States. Bohr himself began to travel more widely,
lecturing in many European countries and in Canada and the United States.
At this time,
more than any of his contemporaries, Bohr stressed the tentative and
symbolic nature of the atomic models that were being used, since he was
convinced that even more radical changes in physics were still to come. In 1924
he was ready to consider the possibility that the conservation laws for energy
and momentum did not hold exactly on the atomic level but were valid only as
statistical averages. This extreme measure for avoiding the apparently
paradoxical particle-like properties of light soon proved to be untenable and
also unnecessary. During the next few years, a genuine quantum mechanics was
created, the new synthesis that Bohr had been expecting. The new quantum
mechanics required more than just a mathematical structure of calculating; it
required a physical interpretation. That physical interpretation came out of
the intense discussions between Bohr and the steady stream of visitors
to his world capital of atomic physics, discussions on how the new mathematical
description of nature was to be linked with the procedures and the results of
experimental physics.
Bohr expressed the characteristic feature of quantum physics in his principle of complementarity,
which "implies the impossibility of any sharp separation between the
behaviour of atomic objects and the interaction with the measuring instruments
which serve to define the conditions under which the phenomena appear." As
a result, "evidence obtained under different experimental conditions cannot
be comprehended within a single picture, but must be regarded as complementary
in the sense that only the totality of the phenomena exhausts the possible
information about the objects." This interpretation of the meaning of
quantum physics, which implied an altered view of the meaning of physical
explanation, gradually came to be accepted by the majority of physicists. The
most famous and most outspoken dissenter, however, was Einstein.
Einstein greatly
admired Bohr's early work, referring to it as "the highest form of
musicality in the sphere of thought," but he never accepted Bohr's
claim that quantum mechanics was the "rational generalization of classical
physics" demanded for the understanding of atomic phenomena. Einstein and Bohr
discussed the fundamental questions of physics on a number of occasions,
sometimes brought together by a close mutual friend, Paul Ehrenfest, professor
of theoretical physics at the University of Leiden, Neth., but they never came
to basic agreement. In his account of these discussions, however, Bohr
emphasized how important Einstein's challenging objections had been to the
evolution of his own ideas and what a deep and lasting impression they had made
on him.
During the 1930s Bohr
continued to work on the epistemological problems raised by the quantum theory
and also contributed to the new field of nuclear physics. His liquid-drop model of the atomic
nucleus, so called because he likened the nucleus to a liquid droplet, was a
key step in the understanding of many nuclear processes. In particular, it
played an essential part in 1939 in the understanding of nuclear fission (the splitting of
a heavy nucleus into two parts, almost equal in mass, with the release of a
tremendous amount of energy). Similarly, his compound-nucleus model of the atom
proved successful in explaining other types of nuclear reactions.
Bohr's institute continued to be a focal point for theoretical
physicists until the outbreak of World War II. The annual conferences on
nuclear physics as well as formal and informal visits of varied duration
brought virtually everyone concerned with quantum physics to Copenhagen at one
time or another. Many of Bohr's collaborators in those years have
written lovingly about the extraordinary spirit of the institute, where young
scientists from many countries worked together and played together in a
lighthearted mood that concealed both their absolutely serious concern with
physics and the darkening world outside. "Even Bohr," wrote
H.B.G. Casimir, one of the liveliest of the group, "who concentrated more
intensely and had more staying power than any of us, looked for relaxation in
crossword puzzles, in sports, and in facetious discussions."
When Denmark was
overrun and occupied by the Germans in 1940, Bohr did what he could to maintain
the work of his institute and to preserve the integrity of Danish culture
against Nazi influences. In 1943, under
threat of immediate arrest because of his Jewish ancestry and the anti-Nazi
views he made no effort to conceal, Bohr, together with his wife and some other
family members, was transported to Sweden by fishing boat in the dead of night
by the Danish resistance movement. A few days later the British government sent
an unarmed Mosquito bomber to Sweden, and Bohr was flown to England in a
dramatic flight that almost cost him his life. During the next two years, Bohr
and one of his sons, Aage (who later followed his father's career as a
theoretical physicist, director of the institute, and Nobel Prize winner in
physics), took part in the projects for making a nuclear fission bomb. They
worked in England for several months and then moved to Los Alamos, N.M., U.S.,
with a British research team.
Bohr's concern
about the terrifying prospects for humanity posed by such atomic weapons was
evident as early as 1944, when he tried to persuade British prime minister
Winston Churchill and U.S. president Franklin D. Roosevelt of the need for
international cooperation in dealing with these problems. Although this appeal
did not succeed, Bohr continued to argue for rational, peaceful policies,
advocating an "open world" in a public letter to the United Nations
in 1950. Bohr was convinced that free exchange of people and ideas was necessary
to achieve control of nuclear weapons. He led in promoting
such efforts as the First International Conference on the Peaceful Uses of
Atomic Energy, held in Geneva (1955), and in helping to create the European
Council for Nuclear Research (CERN). Among his many honours, Bohr received the
first U.S. Atoms for Peace Award in 1957.
In his last years
Bohr tried to point out ways in which the idea of complementarity could throw
light on many aspects of human life and thought. He had a major influence on
several generations of physicists, deepening their approach to their science
and to their lives. Bohr himself was always ready to learn, even from his
youngest collaborators. He drew strength from his close personal ties with his
coworkers and with his sons, his wife, and his brother. Profoundly
international in spirit, Bohr was just as profoundly Danish, firmly rooted in
his own culture. This was symbolized by his many public roles, particularly as
president of the Royal Danish Academy from 1939 until his death in 1962.
Bohr atomic model
description of the structure of atoms, especially that of
hydrogen, proposed (1913) by the Danish physicist Niels Bohr. The Bohr model of the atom, a radical
departure from earlier, classical descriptions, was the first that incorporated
quantum theory and was the predecessor of wholly quantum-mechanical models. The
Bohr model and all of its successors describe the properties of atomic
electrons in terms of a set of allowed (possible) values. Atoms absorb or emit
radiation only when the electrons abruptly jump between allowed, or stationary,
states. Direct experimental evidence for the existence of such discrete states
was obtained (1914) by the German-born physicists James Franck and Gustav Hertz.
Immediately
before 1913, an atom was thought of as consisting of a tiny positively charged
heavy core, called a nucleus, surrounded by light, planetary negative electrons
revolving in circular orbits of arbitrary radii.
Bohr amended that view of the motion of the planetary electrons to
bring the model in line with the regular patterns (spectral series) of light
emitted by real hydrogen atoms. By limiting the
orbiting electrons to a series of circular orbits having discrete radii, Bohr
could account for the series of discrete wavelengths in the emission spectrum
of hydrogen. Light, he proposed, radiated from hydrogen atoms only when an
electron made a transition from an outer orbit to one closer to the nucleus.
The energy lost by the electron in the abrupt transition is precisely the same
as the energy of the quantum of emitted light.
atom
smallest unit
into which matter can be divided without the release of electrically charged
particles. It also is the smallest unit of matter that has the characteristic
properties of a chemical element. As such, the atom is the basic building block
of chemistry.
Most of the atom
is empty space. The rest consists of a positively charged nucleus of protons
and neutrons surrounded by a cloud of negatively charged electrons. The nucleus
is small and dense compared to the electrons, which are the lightest charged
particles in nature. Electrons are attracted to any positive charge by their
electric force; in an atom, electric forces bind the electrons to the nucleus.
It is easier to
describe an atom mathematically than conceptually, and so physicists have
developed several models to explain its various
characteristics. In some respects, the electrons in an atom behave like
particles orbiting the nucleus. In others, the electrons behave like waves
frozen in position around the nucleus. Such wave patterns, called orbitals,
describe the distribution of individual electrons. The behaviour of an atom is
strongly influenced by these orbital properties, and its chemical properties
are determined by orbital groupings known as shells.
This article
opens with a broad overview of the fundamental properties of the atom and its
constituent particles and forces. A more mathematical and technical discussion
of its structure and nucleus is provided in subsequent sections. Included too
is a historical survey of the most influential concepts about the atom that
have been formulated through the centuries. For additional information pertaining
to nuclear structure and elementary particles, see subatomic particles.
Components and properties of
atoms
Constituent particles and forces
Most matter consists of an
agglomeration of molecules, which can be separated relatively easily.
Molecules, in turn, are composed of atoms joined by chemical bonds that are
more difficult to break. Each individual atom consists of smaller
particles--namely, electrons and nuclei. These particles are electrically
charged, and the electric forces on the charge are responsible for holding the
atom together. Attempts to separate these smaller constituent particles require
ever-increasing amounts of energy and result in the creation of new subatomic
particles, many of which are charged.
As noted at the outset of
this article, an atom consists largely of empty space. The nucleus
is the positively charged centre of an atom and contains most of its mass. It
is composed of protons, which have a positive charge, and neutrons, which have
no charge. These constituent protons and neutrons collectively are called nucleons.
Protons, neutrons, and the electrons surrounding them are long-lived particles
present in all ordinary, naturally occurring atoms. Other subatomic particles
may be found in association with these three types of particles. They can be
created only with the addition of enormous amounts of energy, however, and are
very short-lived.
All atoms are roughly the
same size, whether they have three or 90 electrons. Approximately 50,000,000
atoms of solid matter lined up in a row would measure one centimetre (0.4
inch). A convenient unit of length for measuring atomic sizes is the angstrom
(Å), defined as 10-10 metre. The radius of an atom measures 1-2 Å.
Compared with the overall
size of the atom, the nucleus is even more minute. It is in the same proportion
to the atom as a marble is to a football field. In volume, the nucleus takes up
only 10-14 of the space in the atom--i.e., one part in
100,000,000,000,000. A convenient unit of length for measuring nuclear sizes is
the femtometre (fm), which equals 10-15 metre. The diameter of a
nucleus depends on the number of particles it contains and ranges from about 4
fm for a light nucleus such as carbon to 15 fm for a heavy nucleus such as
lead. In spite of the small size of the nucleus, virtually all the mass of the
atom is concentrated there. The protons are massive, positively charged
particles, whereas the neutrons have no charge and are slightly more massive
than the protons. The fact that nuclei can have anywhere from one to about 250
nucleons accounts for their wide variation in mass. The lightest nucleus, that
of hydrogen, is 1,836 times more massive than an electron, while heavy nuclei
are nearly 500,000 times more massive.
Although electrons exhibit
complicated behaviour within an atom, they are characterized completely by a
few parameters. The intrinsic properties of an electron are its charge, mass,
an internal motion called spin, and magnetic moment. All electrons have
identical properties. As the lightest charged particles in existence, they are
absolutely stable because they cannot decay into smaller units. Their charge
and mass, which are important determinants of atomic properties, are listed in Table
1 . The spin of the electron provides it with a directional orientation.
The electron has a magnetic
moment along its spin axis. (Magnetic moment is a property of a particle,
which, like a compass needle, causes its axis to align in a magnetic field.)
Electrons are subject not only to the electromagnetic force but also to the
force of gravity and the so-called weak interaction, the force primarily
manifested in the radioactive decay of nuclei.
Most properties of
atoms--particularly those associated with chemical bonds, physical forces, and
the properties of bulk matter--depend solely on the behaviour of the electrons
surrounding the nucleus. The chemical properties of an atom depend on the
arrangement of its electrons making up the cloud around the nucleus. The atoms
of one element differ from those of other elements in the number of their
electrons. Also, atoms form molecules by lending and sharing electrons. Some
elements, such as alkali metals, have an electron that is loosely bound to the
nucleus and thus easily removed in chemical reactions; other elements, such as
the noble (or inert) gases, have very tightly bound electrons and little
affinity for other electrons. Electrons that have been freed from their atoms
can cause lightning, and freed electrons driven through wires make up ordinary
electric currents.
The nucleus of an atom is
characterized by the number of protons and neutrons in it. Besides a charge and
mass, the nucleus also may have a spin and a magnetic moment of its own,
depending on the internal arrangement of its protons and neutrons. The forces
between nucleons include the three forces affecting electrons as well as the
so-called strong force, which is much more powerful than any of the others.
Because of the strong force, nuclear binding energies are 1,000,000 times the
binding energies of electrons in atoms. The amounts of energy that can be
released in a transformation of the nucleus are correspondingly larger than the
chemical energies released by a transformation of the electron patterns in
atoms. The protons and neutrons in the nucleus are governed by the laws of
quantum mechanics (see below), which describe the complex internal structure of
the nucleus.
Even the individual protons
and neutrons that make up the nucleus have an internal structure of their own.
The constituents of nucleons are called quarks.
Unlike the particles composing the larger units of matter, the quark cannot be
freed from the nucleon and studied in isolation. The strong force acting
between quarks is so powerful that they can never be completely separated. Any
attempt to probe the substructure of a nucleon releases particles of various
types, but the particles produced contain quarks in fixed combinations, never
single quarks. Particles containing quarks are collectively called hadrons.
Hadrons are classified into two categories: baryons
and mesons.
Baryons, which are composed of three quarks, include protons and neutrons as
their lightest examples. Mesons, which contain two quarks, are largely
responsible for nuclear forces. Except for the nucleons, all such particles
decay in a small fraction of a second after their creation.
There is one more broad
category of subatomic particles, the leptons.
Electrons and neutrinos are leptons. They have no detectable internal
components and may be truly fundamental particles of nature. The neutrino is an
uncharged particle with little or no mass that is created during the
radioactive decay of nuclei.
Properties of atoms
Atomic
number
The single most important
characteristic of an atom is its atomic number, which is defined as the number
of units of positive charge in the nucleus. A neutral atom has an equal number
of protons and electrons, so that the positive and negative charges exactly
balance. The atomic number determines the chemical properties of an atom,
including the kinds of molecules that can be formed and their binding energies.
Hence, the atomic number determines an atom's characteristics as an element.
(An element is composed of atoms with the same atomic number.) Elements found
in nature range from atomic number 1, hydrogen, to atomic number 92, uranium.
In addition, artificial elements with atomic numbers beyond 100 have been
produced.
Atomic mass
number
The total number of nucleons
(both protons and neutrons) in an atom is the atomic mass number, or mass
number. Atoms with the same atomic number but different atomic masses are
called isotopes.
Isotopes have identical chemical properties, yet they can have very different
nuclear properties (see the article isotope).
The nuclear properties of an atom include possible radioactivity (the
propensity to become radioactive in nuclear reactions), magnetic properties,
and weight. The element potassium, for example, has two natural isotopes, 39K
and 40K. They form exactly the same compounds, but 40K is
radioactive and decays into another element. In scientific notation, the
isotope of potassium with 19 protons, 20 neutrons, and a total of 39 nucleons
can be written either as 19K39 or as 39/19K.
Because isotopes have the
same number of protons, all of the isotopes of a given element occupy the same
place in the periodic table of elements. Most elements have stable isotopes.
For example, hydrogen has three isotopes, each with one proton. The nucleus of
ordinary hydrogen is an isolated proton, but the isotope deuterium has a
neutron bound to the proton. Both of these isotopes are stable. The third
hydrogen isotope, tritium, has two neutrons and is radioactive. Radioactive
isotopes can be made for many elements; the more the number of neutrons
deviates from the optimum number for that atomic
mass, the shorter the life of the radioactive isotope.
Atomic
weight
The term atomic weight, or
atomic mass, refers to the mass of a fixed number of atoms of an element. The
standard scientific unit for dealing with atoms in macroscopic quantities is
the mole
(mol), which is defined arbitrarily as the amount of a substance with as many
atoms or other units as there are in 12 grams of the carbon isotope 12C.
The number of atoms in a mole is called Avogadro's
number, the value of which is approximately 6 ![]() 1023.
The atomic mass of an element expressed in daltons, or more commonly atomic
mass units (amu's), is the number of grams in one mole of the element. The amu
is convenient because atomic masses are nearly equal to atomic mass numbers and
therefore are close to integer values.
1023.
The atomic mass of an element expressed in daltons, or more commonly atomic
mass units (amu's), is the number of grams in one mole of the element. The amu
is convenient because atomic masses are nearly equal to atomic mass numbers and
therefore are close to integer values.
Historically, the law for
chemical combination according to molar weights was the primary evidence for
the existence of atoms and molecules. For example, two grams of hydrogen
combine with 16 grams of oxygen to form water. This represents two moles of
hydrogen of atomic weight 1 combining with one mole of oxygen of atomic weight
16. Elements consisting of a mixture of several isotopes may not have an atomic
mass close to an integer, because the mass will be the weighted average of the
different isotopes. An example is chlorine, which has two common isotopes, 35Cl
and 37Cl, and a weighted average mass of 35.5 amu.
Electric
charge
The normal atom is
electrically neutral, meaning that it carries a net electric charge of zero.
Some atoms, however, have lost or gained electrons in chemical reactions or in
collisions with other particles. Atoms with a net charge, from either the gain
or loss of electrons, are called ions.
If a neutral atom loses an electron, it becomes a positive ion; if it gains an
electron, it becomes a negative ion.
The charge on any particle is
a whole multiple of the electron's charge, either positive or negative. The
quarks are an exception to this rule. They have charges of +2/3e
and -1/3e. However, they exist only in groups, and
each group as a whole has an integral multiple of the electron's charge. The
amount of charge in this fundamental unit is equal to 1.6 ![]() 10-19
coulomb. This means that in a current of one ampere--roughly what a 100-watt
light bulb uses in the ordinary 110-volt household circuit--about 6
10-19
coulomb. This means that in a current of one ampere--roughly what a 100-watt
light bulb uses in the ordinary 110-volt household circuit--about 6 ![]() 1018
electrons pass through the wire every second.
1018
electrons pass through the wire every second.
Electron
shells
The behaviour of electrons in
atoms is quite subtle and is governed by the laws of quantum
mechanics. According to these laws, electrons occupy various regions of the
atom in frozen wave patterns called orbitals.
The orbitals are most easily visualized as clouds surrounding the nucleus. The
shape and size of the orbital, and the energy of the electron in it, are
calculated by differential equations. The orbitals vary in shape from smooth and
spherical for the electrons most tightly bound to the nucleus to rather diffuse
and lumpy for the least bound electrons. The hydrogen atom has a single
electron in a spherical cloud. The electron could go into other orbitals, but
it would require additional energy in the atom to do so. The quantum
theory provides that the energy of an atom can only change in definite amounts
called quanta. The different possible states an atom can be in, each with its
own definite energy, are called energy
levels. The light emitted from an atom has specific frequencies associated
with the energy quanta. Energy at the atomic level is commonly expressed in electron
volts (eV). There are 2.26 ![]() 1025
eV in one kilowatt-hour. To remove an electron from an atom requires several
electron volts, depending on the atom. Visible light has a quantum energy of
approximately 2 eV.
1025
eV in one kilowatt-hour. To remove an electron from an atom requires several
electron volts, depending on the atom. Visible light has a quantum energy of
approximately 2 eV.
Each electron in a
multielectron atom has its own orbital, according to a law of quantum mechanics
known as the Pauli
principle. Thus, in atoms with many electrons,
many different kinds of orbitals are occupied. A group of orbitals with the
same or nearly the same energy is called a shell. The pattern of filled and
unfilled shells in each element is different; this variety gives the elements
their distinctive characteristics.
Chemical
behaviour
The chemical behaviour of
atoms depends on the shells of the more loosely bound electrons. The Pauli
principle is responsible for chemical valence,
the principle of chemistry according to which atoms of one element bond
to a definite number of atoms in other elements according to simple counting
rules. If these shells are completely filled, the electrons are tightly bound
and the atom does not readily share or lend its electrons to form chemical
bonds. If there is only one electron in the last shell, it is weakly bound and
the atom can be easily ionized. Examples of these situations are helium, which
has a filled shell and is an inert gas, and lithium, which has one more
electron in the next shell and is a highly reactive metal.
One kind of chemical bond is
the ionic
bond, in which a loosely bound electron from one atom transfers to a deeper
shell in another atom. The two ions are held together by their electrical
forces. Another kind of bond is the covalent
bond. In this situation, the electron clouds of one atom are distorted by
the presence of another atom. In the new cloud pattern, the outer electrons are
more concentrated in the region between the two atoms. Thus, the atoms share
their electrons. This allows atoms of the same element to form chemical bonds,
which could not happen with ionic bonds. Chemical-bond energies typically measure
several electron volts.
Nuclear
properties
Like atoms, nuclei have a
shell structure with the protons and neutrons in orbitals. Nuclei can exist in
states of different energy, but ordinary stable nuclei are always in the most
bound state. The scale of these energies is 1,000,000 times as large as atomic
or chemical energies.
Nuclei can undergo
transformations that affect their binding energies. If a transformation leads
to more tightly bound nuclei, the excess energy will be released in some form.
If one mole of atoms undergoes a nuclear transformation and releases 1,000,000
electron volts (1 MeV) of energy per nucleus, the total energy will be 1011
joules.
Some transformations can take
place spontaneously, and such a process is called radioactivity.
In one form of radioactivity, a neutron in the nucleus is converted to a proton
or vice versa. If an electron is emitted at the same time, the process is known
as beta
radioactivity and the electron is called a beta
ray. In another form of radioactivity, the nucleus disintegrates into one
of lower mass number with the excess nucleons being ejected as a small nucleus.
The small nucleus is commonly helium-4. This process is called alpha
radioactivity, and the emitted helium-4 nuclei are called alpha rays. A
third kind of ray observed in radioactivity is the gamma ray. Such rays are
quanta of light of very high energy that are emitted when the nucleus makes a
transition from one energy state to another of lower energy (see the article radioactivity).
Nuclear transformations also
take place in nuclear reactions, which are the processes that occur when a
nucleus is struck by some external particle. In a fusion reaction, two light
nuclei come together and merge into a single heavier nucleus. Another important
reaction is fission, the division of a nucleus into two roughly equal parts.
Fission can be induced in the heaviest elements by reactions with free
neutrons. Both fusion and fission can release energy by reforming the nuclei so
that their atomic masses are closer to the middle range where nuclei have
maximum binding energy (see the articles nuclear
fission and nuclear
fusion).
from quantum mechanics
Bohr's
theory of the atom
A major contribution to the
subject was made by Niels Bohr
of Denmark, who applied the quantum hypothesis to atomic spectra
in 1913. The spectra of light emitted by gaseous atoms had been studied
extensively since the mid-19th century. It was found that radiation from
gaseous atoms at low pressure consists of a set of discrete wavelengths.
This is quite unlike the radiation from a solid, which is distributed over a
continuous range of wavelengths. The set of discrete wavelengths from gaseous
atoms is known as a line
spectrum, because the image of the linear slit in the spectrometer is a
series of sharp lines. The wavelengths of the lines are characteristic of the
element and may form extremely complex patterns. The simplest spectra are those
of atomic hydrogen and the alkali atoms (e.g., lithium, sodium, and
potassium). For hydrogen, the wavelengths ![]() are
given by the empirical formula
are
given by the empirical formula
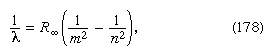
where m and n
are positive integers with n > m and R, known as the Rydberg
constant, has the value 1.0973731 ![]() 107
per metre. For a given value of m, the lines for varying n form a
series. The lines for m = 1, the Lyman
series, lie in the ultraviolet part of the spectrum. Those for m =
2, the Balmer
series, lie in the visible spectrum, and so on.
107
per metre. For a given value of m, the lines for varying n form a
series. The lines for m = 1, the Lyman
series, lie in the ultraviolet part of the spectrum. Those for m =
2, the Balmer
series, lie in the visible spectrum, and so on.
Bohr started
with a model suggested by the New Zealand-born British physicist Ernest
Rutherford. The model was based on the experiments of Hans
Geiger and Ernest
Marsden, who in 1909 bombarded gold atoms with massive, fast-moving alpha
particles; when some of these particles were deflected backward, Rutherford
concluded that the atom has a massive, charged nucleus. In Rutherford's model,
the atom resembles a miniature solar system with the nucleus acting as the Sun
and the electrons as the circulating planets. Bohr made three
assumptions. First, he postulated that, in contrast to classical mechanics,
where an infinite number of orbits is possible, an electron can be in only one
of a discrete set of orbits, which he termed stationary
states. Second, he postulated that the only orbits allowed are those for
which the angular momentum of the electron is a whole number n times (
stands for h/2![]() ).
Third, Bohr assumed that Newton's
laws of motion, so successful in calculating the paths of the planets
around the Sun, also applied to electrons orbiting the nucleus. The force on
the electron (the analogue of the gravitational force between the Sun and a
planet) is the electrostatic attraction between the positively charged nucleus
and the negatively charged electron. With these simple assumptions, he showed
that the energy
of the orbit has the form
).
Third, Bohr assumed that Newton's
laws of motion, so successful in calculating the paths of the planets
around the Sun, also applied to electrons orbiting the nucleus. The force on
the electron (the analogue of the gravitational force between the Sun and a
planet) is the electrostatic attraction between the positively charged nucleus
and the negatively charged electron. With these simple assumptions, he showed
that the energy
of the orbit has the form
![]()
where E0 is
a constant that may be expressed by a combination of the known constants e,
me, and . While in a stationary state, the atom does not give
off energy as light; however, when an electron makes a transition
from a state with energy En to one with lower energy Em,
a quantum of energy is radiated with frequency ![]() ,
given by the equation
,
given by the equation
![]()
Inserting the expression for En
into this equation and using the relation ![]()
![]() =
c, where c is the speed of light, Bohr derived the formula
for the wavelengths of the lines in the hydrogen spectrum, with the correct
value of the Rydberg constant.
=
c, where c is the speed of light, Bohr derived the formula
for the wavelengths of the lines in the hydrogen spectrum, with the correct
value of the Rydberg constant.
Bohr's theory
was a brilliant step forward. Its two most important features have survived in
present-day quantum mechanics. They are (1) the existence of stationary,
nonradiating states and (2) the relationship of radiation frequency to the
energy difference between the initial and final states in a transition. Prior
to Bohr, physicists had thought that the radiation frequency would be
the same as the electron's frequency of rotation in an orbit.
The Bohr
model
The first attempt to
introduce quantum theory to account for the structure of atoms was made by the
Danish physicist Niels Bohr in 1913. He asserted that the
electron in a hydrogen atom occupies one of an array of discrete (but infinite
in number) orbits, each orbit being progressively farther from the nucleus and
labeled with an integer n = 1, 2, . . . . This integer is an example of
a quantum number, which in general is an integer (in some cases, a
half-integer) that labels the state of a system and which, through an
appropriate formula, determines the values of certain physical properties of
the system. By matching the centrifugal effect of the electron's motion in its
orbit to the electrostatic attraction of the nucleus for the electron, Bohr
was able to find a relation between the energy of the electron and the quantum
number of its orbit. The result he obtained was in almost perfect agreement
with the observed values of the energy levels of a hydrogen atom that had
previously been obtained by spectroscopic methods.
Bohr's triumph
was the first apparently successful incorporation of quantum
theoretical ideas into the description of a mechanical system. The numerical
success of the model has turned out to be coincidental, however, and Bohr's
model is now regarded as no more than a historically important step in the
evolution of quantum mechanics. The cracks in its validity were noted quite
soon after its introduction. Thus, it was remarked that Bohr had not
really derived the existence of discrete orbits from more fundamental principles
but had merely imposed them on the model. Furthermore, all attempts to extend
his theory to atoms that consisted of more than one electron (helium, with two
electrons, for instance) utterly failed. Although the model was augmented by
more elaborate specifications of the orbits (most notably, first, by allowing
for elliptical orbits and introducing a second quantum number to specify the
elongation of the ellipse and, second, by allowing for the effects of
relativity), the failure to generalize to many-electron atoms remained a fatal
flaw.
